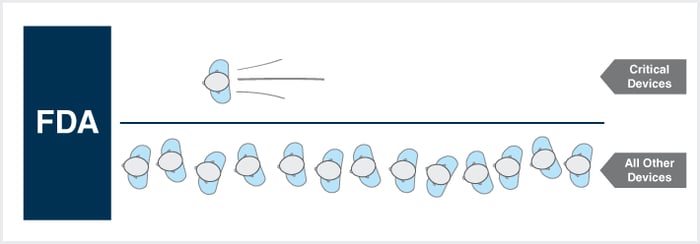
On April 15th, the Food and Drug Administration will begin accepting applicants through its new Expedited Access Premarket Approval Application for Unmet Medical Needs for Life Threatening or Irreversibly Debilitating Diseases or Conditions (“Expedited Access PMA” or “EAP”). Read more