The topic of diversity and inclusion in the workplace and in research has been discussed for decades. The need to improve these initiatives has gained even more attention as recent events and studies have highlighted many disparities such as those in healthcare. Many factors contribute to disparities in healthcare and clinical research, all of which lead to very real consequences. Read more
usability testing
Usability Validation of Sterile Packaging
/ in usability testing , HFES , validation / by Design ScienceMake sure to join us May 18th to May 21st for the virtual HFES Healthcare Symposium! Not only will we be hosting an interactive virtual workshop but four of our experts will also be presenting their HFES posters. Interested in usability validation? Join us to listen to one of our Senior Human Factors Engineers, David Grosse-Wentrup, while he shares his methods for the usability validation of. Read more
Planning for the Unplanned: Learnings from Usability Studies Can Inform the Design of Emergency Use Medical Products
/ in simulated use , tips , usability testing / by Design ScienceOur very own Research Director, Angela Muriset, will be presenting her HFES poster at this year’s virtual HFES Healthcare Symposium. Join us to get Angela’s insight on achieving simulation realism when evaluating medical devices and combination products for emergency scenarios. When stress is a real-life factor how do you address that in a human factors study? Join us May 18th to 21st at the. Read more
7 Practical Tips to Get More Value from Eye Tracking Research
/ in research , study design , usability testing , data visualization , eye tracking , human factors , innovative technology , medical product design , user research / by Ranjan NayyarWorking on numerous eye tracking studies over the last two years, I feel I have gained the authority to say a word or two on eye tracking research. The research method is critical to our work at Design Science and has found itself to be ever more promising our clients and to us than ever before. Using eye tracking successfully in the field requires both a firm grasp on the theory and practical. Read more
A Guide to Trainings
/ in training , usability testing , human factors , news / by Christina SAs a recent addition to the Design Science team, I know that there is a steep learning curve when it comes to the specific tools and skills needed for usability testing. There’s a lot to learn, from the nuances of interview styles and follow-up questioning to the requirements of study documentation and reporting. Thankfully, an effective training program can make all the difference when. Read more
Testing Abroad: Lessons from the Field
/ in study design , tips , travel , usability , usability testing , abroad , HF , human factors , news / by Christina SConducting usability studies abroad is an exciting opportunity for researchers. For those who get to travel to new places, you get to see another part of the world, eat new foods, and learn new customs. There are many resources available with tips and suggestions on how to plan an international trip—but planning a vacation abroad and planning a usability study abroad are two different things. Read more
MDO Article: The Value Of Eye-Tracking Software In Medical Device Usability Testing
/ in usability testing , eye tracking , human factors , MDO , meddeviceonline , medical product design , news / by Christina SWhen conducting usability testing, moderators rely heavily upon observations and interviews in order to evaluate medical devices. However, as Data & Systems Analyst Mahajabin Rahman points out in Design Science’s most recent article for MedDeviceOnline, the use of eye-tracking software can support these methods with objective, real-time data. Read more
MDO Article: Staying Dry: How To Limit Wet Injections In Auto-Injector Design
/ in usability testing , human factors , MDO , meddeviceonline , medical product design , news / by Christina SIn Design Science’s most recent guest column for MedDeviceOnline, Analyst Bryon Calawa examines the challenges that researchers face when evaluating auto-injectors in usability studies. Read more
MDO Article: "Achieving Realism In Human Factors Work: How To Stay Out Of Fantasy Land"
/ in usability testing , FDA , healthcare , human factors , MDO , meddeviceonline , news / by Christina SDesigning usability studies that are "representative" is not only necessary for valid data, it's also an FDA requirement. Chad Uy shares some methods for maintaining realism to ensure representative usability studies in his recent article for Med Device Online. Read more
MDO Article: “How To Know You've Passed Validation Testing (And What To Do If You Haven't)”
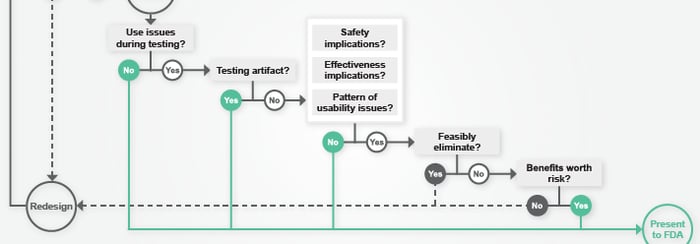
/ in usability testing , design science , FDA , human factors , MDO , meddeviceonline , medical product design , news , presentations / by Christina SUnderstanding the ramifications of less-than-perfect validation testing results can be complex. In his recent article for Med Device Online, Peter Sneeringer presents 6 questions to help companies decide if errors seen during testing indicate a likelihood that the FDA will reject their submission. Read more