Confusion and mistrust can cloud a lot of the conversations about the COVID-19 vaccines. From safety to sample size, many people have expressed concerns about how quickly the vaccines were developed, especially in comparison to more traditional vaccines, and whether corners were cut in the process. Read more
fda
An Introduction to HIPAA Compliant Software
/ in software , cybersecurity , FDA , HIPAA , IT , news / by Christina SIn today’s fast-paced business world, cloud-based services are taking over. These services are identified with terms like “platform as a service” (PaaS), “software as a service” (SaaS), and even “infrastructure as a service” (IaaS). Each of these refers to a service based in data centers that you access through the internet—as opposed to local servers in your work environment. It is only natural. Read more
MD+DI Article: “Root Cause Analysis: Adventures in Medical Device Usability”
/ in root cause , root cause analysis , FDA , guest article , MDDI , news , RCA / by Christina SWhat is Root Cause Analysis? What is its unique role in formative vs. summative testing? How should it be planned for before usability testing even starts? Read more
Listen: Podcast on Human Factors & Usability Testing for TechnologyAdvice
/ in technologyadvice , contextual inquiry , ethnography , expert interview , FDA , news , prototyping / by Christina SCheck out Design Science founder Steve Wilcox's thoughts on ethnography, prototyping, the FDA, and more through TechnologyAdvice’s TA Expert Interview Series. Read more
MDO Article: "Achieving Realism In Human Factors Work: How To Stay Out Of Fantasy Land"
/ in usability testing , FDA , healthcare , human factors , MDO , meddeviceonline , news / by Christina SDesigning usability studies that are "representative" is not only necessary for valid data, it's also an FDA requirement. Chad Uy shares some methods for maintaining realism to ensure representative usability studies in his recent article for Med Device Online. Read more
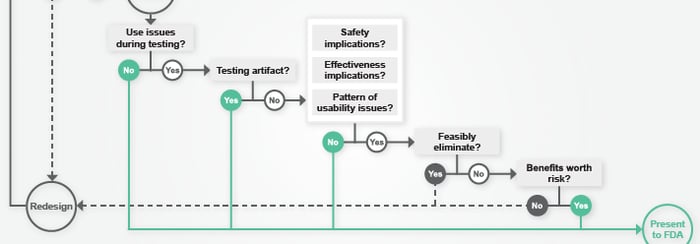
MDO Article: “How To Know You've Passed Validation Testing (And What To Do If You Haven't)”
/ in usability testing , design science , FDA , human factors , MDO , meddeviceonline , medical product design , news , presentations / by Christina SUnderstanding the ramifications of less-than-perfect validation testing results can be complex. In his recent article for Med Device Online, Peter Sneeringer presents 6 questions to help companies decide if errors seen during testing indicate a likelihood that the FDA will reject their submission. Read more
FDA to Accept Applications for Expedited Medical Device Approval
/ in usability testing , user-centered design , design science , EAP , Expedited Access PMA , FDA , guidance , healthcare , human factors , industrial design , medical product design , news / by Christina SOn April 15th, the Food and Drug Administration will begin accepting applicants through its new Expedited Access Premarket Approval Application for Unmet Medical Needs for Life Threatening or Irreversibly Debilitating Diseases or Conditions (“Expedited Access PMA” or “EAP”). Read more