COVID-19 Vaccine: Demystifying Operation Warp Speed
/ in design science , FDA , healthcare , information , covid19 , vaccine / by Michelle KannegiserConfusion and mistrust can cloud a lot of the conversations about the COVID-19 vaccines. From safety to sample size, many people have expressed concerns about how quickly the vaccines were developed, especially in comparison to more traditional vaccines, and whether corners were cut in the process.
At Design Science, we understand that sometimes too much information can become confusing and distract us from its purpose. In an effort to demystify and clarify the COVID-19 vaccines’ path to the public, we examined their development process and any external factors that supported its rapid approval.
Before we do that, it is important to understand the rigorous process every vaccine (including the COVID-19 vaccines) goes through before the U.S. Food and Drug Administration (FDA) determines that it is safe and effective for use in the United States1.
 Research and Discovery Stage
Research and Discovery Stage
Scientists study the virus and how it behaves to theorize and test possible vaccines components.
 Pre-clinical
Pre-clinical
Before the vaccine can be tested in people, it is tested in animals to observe its efficacy and to make a preliminary conclusion about how likely it is to be safe and effective in humans.
 Investigational New Drug (IND) Application
Investigational New Drug (IND) Application
The FDA reviews pre-clinical research to determine if the drug is reasonably safe to test on people.
 Clinical Development, Phase 1
Clinical Development, Phase 1
Human testing begins with a small sample size of about 20 to 100 volunteers. This initial human testing is focused on monitoring the new vaccine’s safety, especially in regards to adverse reactions with different dose strengths, as well as efficacy.
 Clinical Development, Phase 2
Clinical Development, Phase 2
Human testing progresses with a larger sample size of several 100 participants. During these trials, there is typically a control group to allow scientists to collect data about short-term side effects, safety, risks, doses, and immune responses.
 Clinical Development, Phase 3
Clinical Development, Phase 3
This is the final and largest phase of human testing, with a typical sample size around 30,000 participants. In this phase, scientists compare incidences of disease in those who receive the vaccine to those who receive a placebo, thus allowing them to determine the vaccine’s effectiveness.
 Assessment of Manufacturing
Assessment of Manufacturing
This phase produces proof that there is a manufacturer in place that can reliably and consistently produce the vaccine.
 Biologics License Application (BLA)
Biologics License Application (BLA)
Data from each phase of development and manufacturing is collected and submitted to the FDA. Receiving a BLA means the vaccine has been rigorously tested and has been proven to be safe and effective according to the FDA’s guidelines2.
Figure 1 below shows two versions of this development process. The first chart illustrates the development process of a vaccine on a typical timeline, and the second chart overlays the timeline of the COVID-19 vaccines so we can easily compare the two timelines.
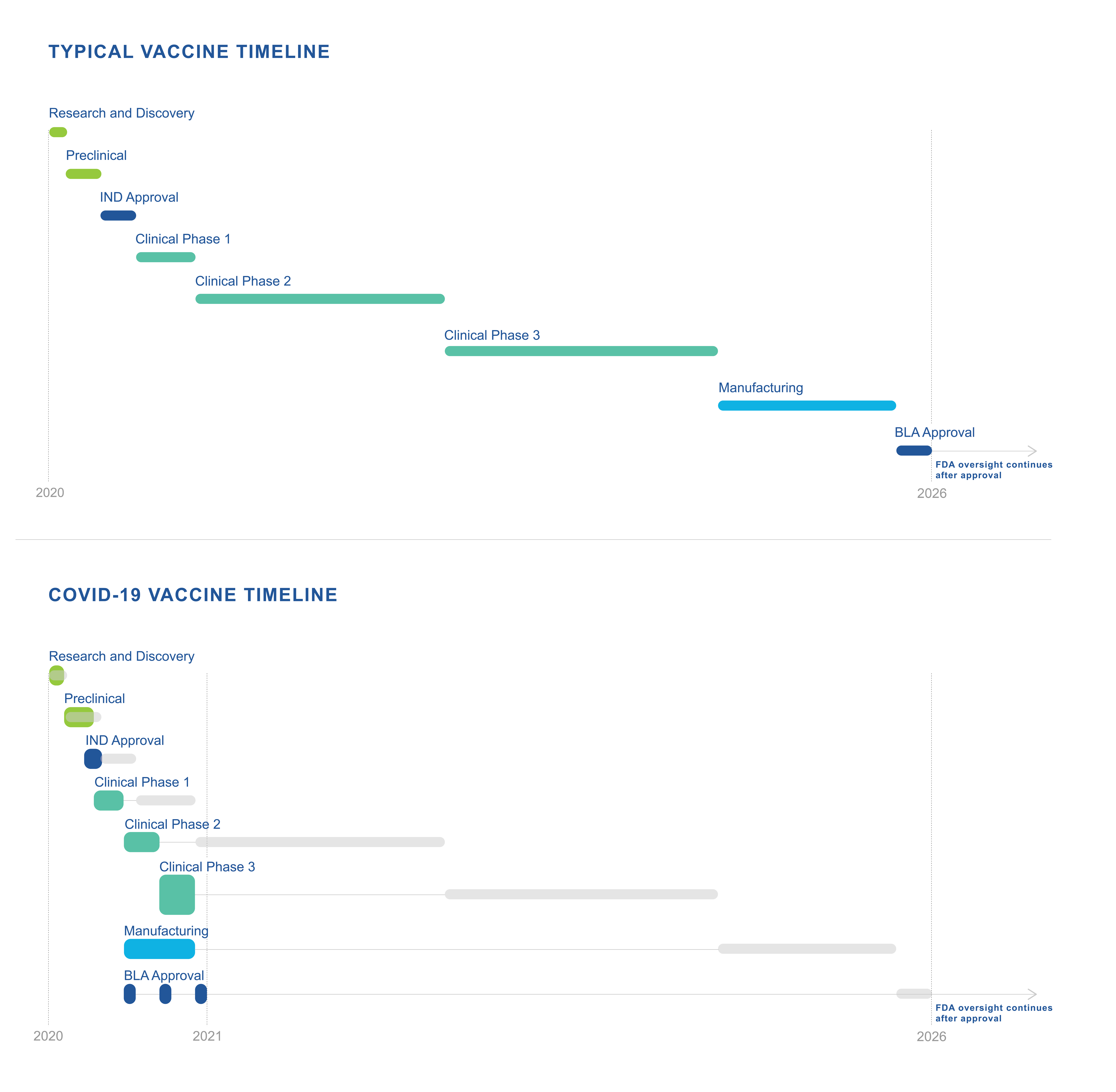 Figure 1: Typical timeline for vaccine development vs. COVID-19 vaccine development
Figure 1: Typical timeline for vaccine development vs. COVID-19 vaccine development
To properly compare the two processes illustrated in Figure 1, let’s begin by defining two terms associated with the COVID-19 vaccines: Operation Warp Speed and Fast Track.
Operation Warp Speed is the partnership between the U.S. Department of Health and Human Services, the U.S. Department of Defense, and private companies to develop, produce, and distribute vaccines for COVID-193. Their main goal was to facilitate the development of vaccines on a shorter timeline without sacrificing quality or safety by:
- Utilizing existing technology: Using existing technologies, such as platforms already developed for other diseases, allowed Research and Discovery to discover potential vaccines sooner.
- Immediately beginning development after discovering the viral genome sequence: With support of the federal government and increased communication with the FDA, companies moved straight from Research and Discovery to Preclinical without risk or question of funding.
- Facilitating large-scale manufacturing: As shown in Figure 1, manufacturing typically does not begin until a vaccine is deemed successful. But in the case of the COVID-19 vaccines, the government facilitated large scale manufacturing earlier, saving a lot of time at the end of the development process while removing the risk from the companies involved.
- Financing by the federal government: Financing affects each phase of development as it allows companies to allocate more resources to accelerate development. Specifically, in Clinical Development-Phase 3 of the COVID-19 process, companies were able to simultaneously conduct multiple large-scale clinical trials to test the same sample size in about an eighth of the time.
Fast track is a designation given by the FDA to certain projects to facilitate the development and expedite the review of drugs intended to treat serious conditions4. The purpose of this designation is to get critical new drugs to the patients earlier and is done by increasing communication with the FDA to assure questions and issues are resolved quickly throughout the development process. This includes:
- Holding more frequent check-ins with the FDA, including communication about trial design and biomarkers: Having the FDA monitor the development and collection of appropriate data throughout the development process ensures that there are no surprises when the agency reviews the IND or BLA, which allows the review process to be completed faster.
- Accelerated review process: A typical review process of a BLA submission could take up to 10 months, but with the FDA prioritizing COVID-19 vaccines the review process was accelerated and split into multiple, smaller review cycles allowing manufacturers to submit sections of their application for review throughout the process instead of compiling it all at the end for submission.
As illustrated above, despite some citizens’ concerns, the COVID-19 vaccines still went through the same rigorous research process to prove their safety and effectiveness, though more quickly than the typical vaccine. We would like to invite you to leave a comment if you have questions or visit our resource links below!
Resources:
2 General Principles for the Development of Vaccines to Protect Against Global Infectious Diseases
3 Operation Warp Speed: US Department of Defense
FDA: Development and Licensure of Vaccines to Prevent COVID-19
Share this entry
-
Share on Facebook
Share on Facebook
-
Share on Twitter
Share on Twitter
-
Share on Google+
Share on Google+
-
Share on Linkedin
Share on Linkedin
-
Share by Mail
Share by Mail