When conducting usability testing, moderators rely heavily upon observations and interviews in order to evaluate medical devices. However, as Data & Systems Analyst Mahajabin Rahman points out in Design Science’s most recent article for MedDeviceOnline, the use of eye-tracking software can support these methods with objective, real-time data. Read more
mdo
MDO Article: Staying Dry: How To Limit Wet Injections In Auto-Injector Design
/ in usability testing , human factors , MDO , meddeviceonline , medical product design , news / by Christina SIn Design Science’s most recent guest column for MedDeviceOnline, Analyst Bryon Calawa examines the challenges that researchers face when evaluating auto-injectors in usability studies. Read more
MDO Article: "Achieving Realism In Human Factors Work: How To Stay Out Of Fantasy Land"
/ in usability testing , FDA , healthcare , human factors , MDO , meddeviceonline , news / by Christina SDesigning usability studies that are "representative" is not only necessary for valid data, it's also an FDA requirement. Chad Uy shares some methods for maintaining realism to ensure representative usability studies in his recent article for Med Device Online. Read more
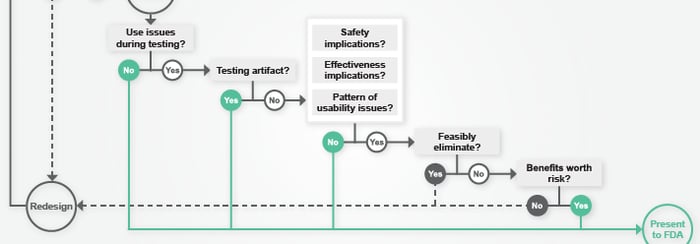
MDO Article: “How To Know You've Passed Validation Testing (And What To Do If You Haven't)”
/ in usability testing , design science , FDA , human factors , MDO , meddeviceonline , medical product design , news , presentations / by Christina SUnderstanding the ramifications of less-than-perfect validation testing results can be complex. In his recent article for Med Device Online, Peter Sneeringer presents 6 questions to help companies decide if errors seen during testing indicate a likelihood that the FDA will reject their submission. Read more
MDO Article: "4 Proposals To Accelerate The Growth of mHealth"
/ in usability , user-centered design , app design , design science , healthcare , human factors , interaction design , MDO , meddeviceonline , mHealth , mobile app , news / by Christina SWhat 4 things should app developers, traditional medical device and pharmaceutical companies, and regulators do to accelerate the growth of mHealth?Check out Nicholas Araujo's proposals, featured on Med Device Online's website. Read more