Design Science Attends 2016 HFES Symposium: Shaping the Future
/ in Shaping the Future , Symposium , conference , HF , HFES , human factors , news / by Christina SThe recent HFES Symposium in San Diego brought together human factors’ professionals from across the globe to discuss the future of the field. In line with the theme of this year’s conference—Shaping the Future—attendees presented on new lines of research, applications of technology, and opportunities to enhance the safety of medical devices and health care practices.
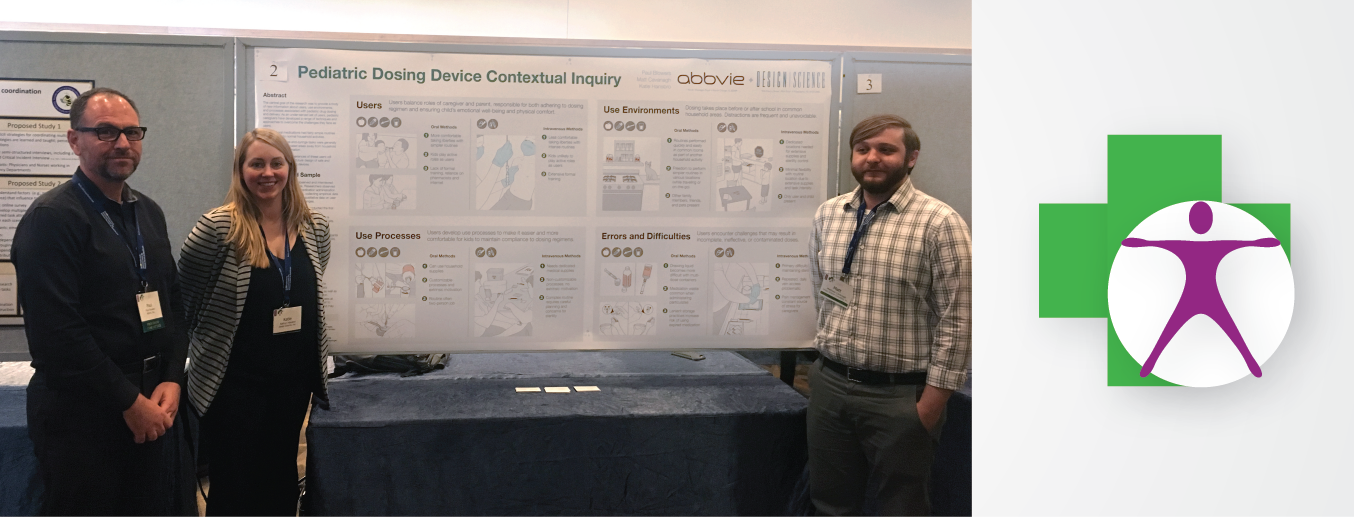
Design Science was represented well at the conference, with Steve Wilcox, Katie Hansbro, Peter Sneeringer, Lindsay Carrabine, and Matt Cavanagh all in attendance. Steve presented about the effect of the Internet of Things on healthcare with Hugh Dubberly of Dubberly Design Office. Peter and Lindsay co-presented on ways that eye-tracking can be used in usability testing. Matt and Katie joined with AbbVie's Paul Blowers to present a poster summarizing a contextual inquiry into pediatric dosing routines. Importantly, the conference also gave human factors staff at FDA the chance to update those in attendance on critical developments in the 2016 guidance.
- The CDRH HF Pre-market team was very approachable and clear about their expectations for 510K submissions.
- Irene Chan from DMEPA echoed much of what the CDRH team said, pointing to improved alignment between the centers in the coming year.
- A main theme for HF submissions is “Building the Story.” Focusing on the validation testing results in your HFE/UE Report shows readers only the final act of the story. Instead, show FDA all of the work you’ve done to design your device well; then let the validation test support your design.
- In its eyes, use errors have the potential to cause harm. Critical tasks are those on which a use error could occur. FDA wants to see all critical tasks tested in a validation study.
- FDA is creating guidance for IFU creation and is standardizing its IFU review process. Stay tuned for more info.
Share this entry
-
Share on Facebook
Share on Facebook
-
Share on Twitter
Share on Twitter
-
Share on Google+
Share on Google+
-
Share on Linkedin
Share on Linkedin
-
Share by Mail
Share by Mail