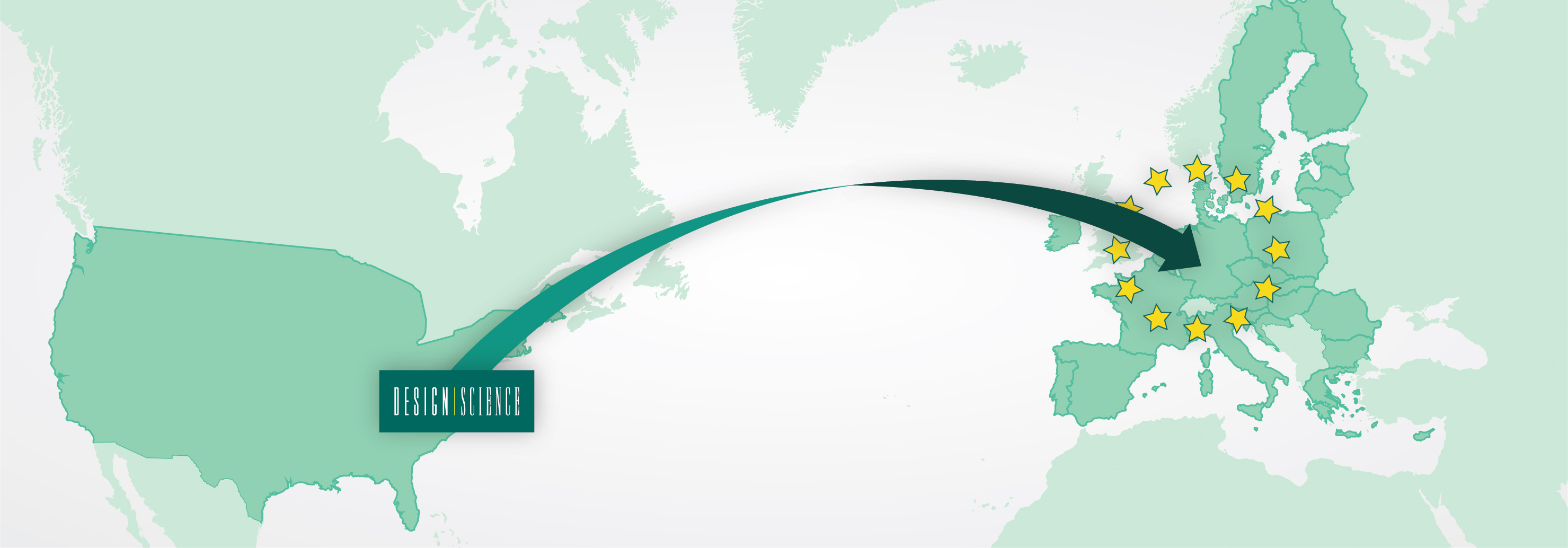
Design Science Expands to the EU
/ in announcement , global , news / by Christina SMedical devices, combination products, and pharmaceuticals are global products, created by multi-national companies, intended for unique national markets.
These markets, especially in the U.S. and EU which are at the forefront of shaping the medical world, differ greatly regarding their regulatory requirements and approaches to usability engineering. Moreover, both markets are individually dynamic, with updating standards, new developments and trends, as well as changing political conditions.
 However, many similarities do exist between these markets. When facing the usability lifecycle of a global product, numerous aspects and activities of the usability engineering process can be transferred and adapted for multiple markets to minimize overhead and cost during the development.
However, many similarities do exist between these markets. When facing the usability lifecycle of a global product, numerous aspects and activities of the usability engineering process can be transferred and adapted for multiple markets to minimize overhead and cost during the development.
Understanding these differences and seeing synergies calls for a global perspective with an eye for national specifics. It takes a company that shares its clients’ global approach to support and guide them through the challenges of usability engineering for international products.
To keep the finger on the pulse of the time and ahead of the industry, Design Science has now contracted its first full-time employee in Europe. In preparation for Design Science’s upcoming European branch office, David, who has been a part of DS’ U.S. team for over a year, relocated to Germany to advance Design Science’s presence in central Europe.
Advancing a global approach for one universal goal: fitting products to people.
Share this entry
-
Share on Facebook
Share on Facebook
-
Share on Twitter
Share on Twitter
-
Share on Google+
Share on Google+
-
Share on Linkedin
Share on Linkedin
-
Share by Mail
Share by Mail