Verification Vs. Validation: Twin Cities in the Land of Design Control
/ in study design , usability , user-centered design , news , validation , verification / by Christina SVerification and validation are two critical elements of product development. One informs the elements of a product’s design, while the other looks at those design elements in the hands of users. Although often used synonymously, these alliterative terms sit on opposite sides of the product development life cycle.
If design control is a country, then verification and validation are twin cities in that nation;  they are closely related and their borders can seem nearly indistinguishable at times. What’s more, there is often movement and shared resources between them; but, importantly, they are unique.
they are closely related and their borders can seem nearly indistinguishable at times. What’s more, there is often movement and shared resources between them; but, importantly, they are unique.
Understanding the subtle differences between verification and validation is crucial to providing clients with an accurate picture of the design control process. The U.S. code of federal regulations, 21 CFR 820.30, and its international counterpart, ISO 13485 Section 7, set forth requirements of the design control process as it is related to medical devices.
- Design verification ‘means confirming by examination and provision of objective evidence that specified requirements have been fulfilled.’ In other words, ‘Did you build what you said you would build?’
- In contrast, design validation ‘means establishing by objective evidence that device specifications conform to user needs and intended use.’ That is, ‘Does what you built do what the user needs it to do?’
 Verification is a paper exercise that ensures each requirement in the design inputs is accounted for in the design outputs. It documents how a product went from concept to construct. For example, if a design input requires a continuous glucose monitor to have a screen for patients to check their glucose levels, verification looks at the design output (e.g. prototype) and verifies that there is, indeed, a screen with the specified dimensions. On the other hand, validation is a practical test that ensures the product’s specifications meet user needs. It validates that a design element works in the hands of intended users, as demonstrated through a set of tasks that put a verified design element in action. Thus, a design input can easily be verified, but not validated—i.e., there is some disconnect between form and function.
Verification is a paper exercise that ensures each requirement in the design inputs is accounted for in the design outputs. It documents how a product went from concept to construct. For example, if a design input requires a continuous glucose monitor to have a screen for patients to check their glucose levels, verification looks at the design output (e.g. prototype) and verifies that there is, indeed, a screen with the specified dimensions. On the other hand, validation is a practical test that ensures the product’s specifications meet user needs. It validates that a design element works in the hands of intended users, as demonstrated through a set of tasks that put a verified design element in action. Thus, a design input can easily be verified, but not validated—i.e., there is some disconnect between form and function.
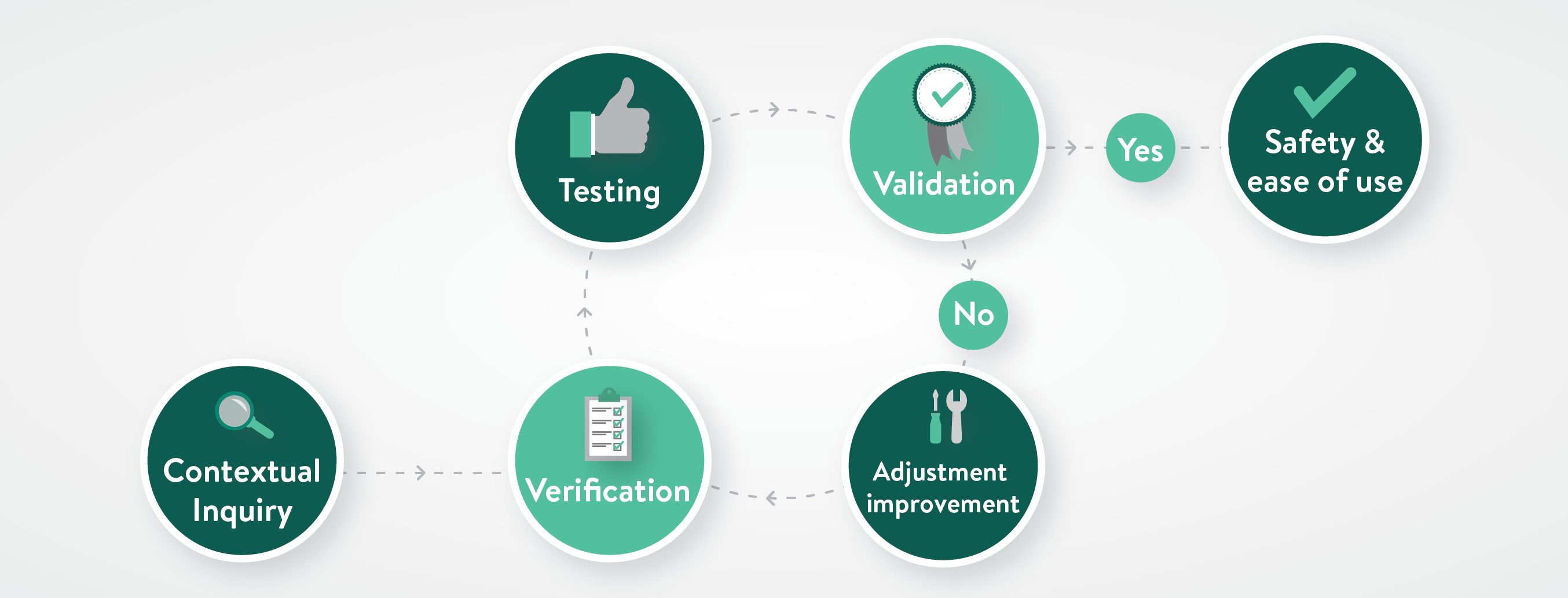
 Therefore, verification and validation are iterative: verify, validate, and repeat, as necessary, until the device is demonstrably safe and easy to use. Design Science fulfills important roles in this process. In the early stages, we contribute to the development of design inputs by conducting contextual inquiry research for our clients. Contextual inquiry findings become the objective evidence on which to base user-related design inputs for new products. For example, we might observe that, while an existing surgical tool is designed for both left- and right-handed use, its power supply cord gets tangled or ungainly when actually used by a left-handed surgeon. Such data might suggest a requirement for cordless power supply in the design inputs of a new device.
Therefore, verification and validation are iterative: verify, validate, and repeat, as necessary, until the device is demonstrably safe and easy to use. Design Science fulfills important roles in this process. In the early stages, we contribute to the development of design inputs by conducting contextual inquiry research for our clients. Contextual inquiry findings become the objective evidence on which to base user-related design inputs for new products. For example, we might observe that, while an existing surgical tool is designed for both left- and right-handed use, its power supply cord gets tangled or ungainly when actually used by a left-handed surgeon. Such data might suggest a requirement for cordless power supply in the design inputs of a new device.
Once a product has reached the validation stage of development—usually after several rounds of formative usability testing—we conduct testing to validate that user needs and requirements have been met. For instance, our client may set a requirement for their new auto-injector which necessitates 95% of doses be administered within 5 seconds of injection. We might test 100 injections in a simulated-use environment, measuring the dispensation time for each dose. If 95 or more of the doses are administered within 5 seconds, we can say the requirement is validated.
As elements of the product development process, verification and validation play crucial, yet distinct, roles. Each occupies its own space, but these spaces have important touch points where one process cycles into the other.
This post was edited by Matthew Cavanagh.
Share this entry
-
Share on Facebook
Share on Facebook
-
Share on Twitter
Share on Twitter
-
Share on Google+
Share on Google+
-
Share on Linkedin
Share on Linkedin
-
Share by Mail
Share by Mail