The HIMSS 2017 conference is “the year's largest and most important healthcare IT conference in the United States” and brings together executives, clinicians, IT professionals, and more for a week of networking, knowledge-sharing, and forward-thinking. Read more
human factors
5 Tips for Your First Study in a Necropsy Lab
/ in tips , usability , cadaver , HF , human factors , medical product design , news / by Christina SWhy Use Cadavers? The study of human anatomy using cadavers dates back to roughly 300 BC, when the Greek physician Herophilus started to use dissection to understand human anatomy. Since then, cadavers have served as a major aid to education and research. For medical students learning anatomy and surgeons perfecting their instrument techniques, cadavers provide physical training materials. What's. Read more
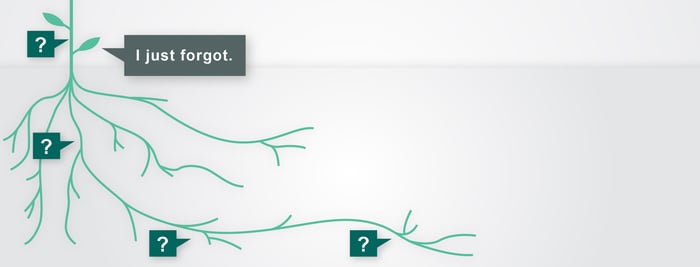
Getting to the Root
/ in root cause , usability study , user-centered design , analysis , HF , how-to , human factors , news / by Christina SHuman behavior can be hard to predict and even harder to explain. Study participants will often manage to do similar things in different ways, and they’ll rarely work with the same tools with equal proficiency. Read more
A Guide to Trainings
/ in training , usability testing , human factors , news / by Christina SAs a recent addition to the Design Science team, I know that there is a steep learning curve when it comes to the specific tools and skills needed for usability testing. There’s a lot to learn, from the nuances of interview styles and follow-up questioning to the requirements of study documentation and reporting. Thankfully, an effective training program can make all the difference when. Read more
Design Science Attends 2016 HFES Symposium: Shaping the Future
/ in Shaping the Future , Symposium , conference , HF , HFES , human factors , news / by Christina SThe recent HFES Symposium in San Diego brought together human factors’ professionals from across the globe to discuss the future of the field. In line with the theme of this year’s conference—Shaping the Future—attendees presented on new lines of research, applications of technology, and opportunities to enhance the safety of medical devices and health care practices. Read more
Testing Abroad: Lessons from the Field
/ in study design , tips , travel , usability , usability testing , abroad , HF , human factors , news / by Christina SConducting usability studies abroad is an exciting opportunity for researchers. For those who get to travel to new places, you get to see another part of the world, eat new foods, and learn new customs. There are many resources available with tips and suggestions on how to plan an international trip—but planning a vacation abroad and planning a usability study abroad are two different things. Read more
MDO Article: The Value Of Eye-Tracking Software In Medical Device Usability Testing
/ in usability testing , eye tracking , human factors , MDO , meddeviceonline , medical product design , news / by Christina SWhen conducting usability testing, moderators rely heavily upon observations and interviews in order to evaluate medical devices. However, as Data & Systems Analyst Mahajabin Rahman points out in Design Science’s most recent article for MedDeviceOnline, the use of eye-tracking software can support these methods with objective, real-time data. Read more
MDO Article: Staying Dry: How To Limit Wet Injections In Auto-Injector Design
/ in usability testing , human factors , MDO , meddeviceonline , medical product design , news / by Christina SIn Design Science’s most recent guest column for MedDeviceOnline, Analyst Bryon Calawa examines the challenges that researchers face when evaluating auto-injectors in usability studies. Read more
MDO Article: "Achieving Realism In Human Factors Work: How To Stay Out Of Fantasy Land"
/ in usability testing , FDA , healthcare , human factors , MDO , meddeviceonline , news / by Christina SDesigning usability studies that are "representative" is not only necessary for valid data, it's also an FDA requirement. Chad Uy shares some methods for maintaining realism to ensure representative usability studies in his recent article for Med Device Online. Read more
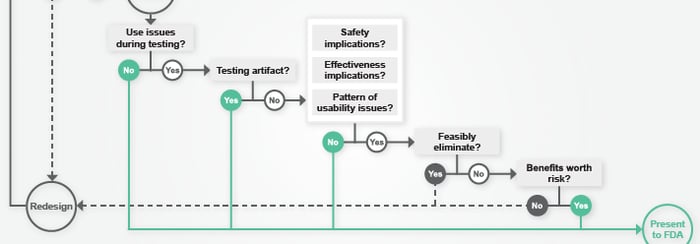
MDO Article: “How To Know You've Passed Validation Testing (And What To Do If You Haven't)”
/ in usability testing , design science , FDA , human factors , MDO , meddeviceonline , medical product design , news , presentations / by Christina SUnderstanding the ramifications of less-than-perfect validation testing results can be complex. In his recent article for Med Device Online, Peter Sneeringer presents 6 questions to help companies decide if errors seen during testing indicate a likelihood that the FDA will reject their submission. Read more